| Aim | Determination of pH using pH paper/universal indicator |
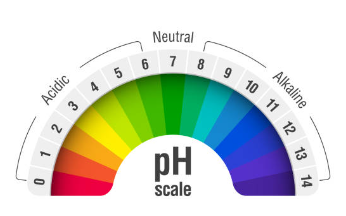
| Theory | What is pH? pH refers to a quantitative measure of the concentration of hydrogen ions in a solution, which is used to determine whether the solution is acidic or alkaline. If a solution has a pH value less than 7, it is classified as acidic. If a solution has a pH value greater than 7, it is classified as alkaline. If a solution has a pH value of 7, it is classified as neutral. What is the pH scale? The pH scale is a numerical scale that ranges from 0 (extremely acidic) to 14 (extremely alkaline), and it is used to indicate the acidity or alkalinity of a solution. The values on the scale are used to determine the concentration of hydrogen ions. What is pH paper? pH paper is a simple and quick method to determine whether a solution is acidic, alkaline, or neutral. By dipping the pH paper into the solution to be tested, a color change occurs on the paper. This color change is then compared to a standard pH color chart. In addition to pH paper, universal indicator paper or universal indicator solution can also be used. What is universal indicator? A universal indicator is a mixture of pH indicator solutions that is designed to measure the pH of solutions across a wide range of values. A drop of solution is placed on the universal pH indicator paper, and the color that develops on the paper is then compared to a standard pH color chart. |
| Chemical Required | Dilute hydrochloric acid Dilute NaOH solution Dilute ethanoic acid solution Lemon juice Water Dilute hydrogen carbonate solution |
| Apparatus Required | pH paper/universal indicator Dropper Test tubes Stirring rod Disposable pipettes |
| Procedure | 1 . Label the test tubes with the name of the sample to be tested. 2 . Take 2 ml of each sample in a separate test tube using a disposable pipette. 3 . Dip a pH paper or universal indicator in each test tube for a few seconds. 4 . Observe the color change on the pH paper or universal indicator and compare it with the color chart provided with the pH paper or 5 . universal indicator. 6 . Record the pH value of each sample in a table. 7 . Rinse the pH paper or universal indicator with distilled water before testing the next sample. 8 . Repeat the experiment for each sample. |
| Result |  |
| Discussion | The pH of a solution indicates its acidity or basicity. A pH value of 7 indicates neutrality, while pH values below 7 indicate acidity and pH values above 7 indicate basicity. In this experiment, we have used pH paper or universal indicator to determine the pH of different samples. Dilute hydrochloric acid is highly acidic and has a pH of approximately 1, while dilute NaOH solution is highly basic and has a pH of approximately 13. Dilute ethanoic acid has a pH of 3-4, making it weakly acidic. Lemon juice, which contains citric acid, has a pH of 2-3, indicating it is more acidic than ethanoic acid. Water is neutral, and its pH value is 7. Dilute hydrogen carbonate solution has a pH of 8-9, making it weakly basic. |
| Aim | Studying the Properties of Acids and Bases (HCl & NaOH) Using Litmus Solution and Zinc Metal |
| Theory | What is acid? Chemical species which donate protons or release H+ ions when dissolved in water are called acid. They turn blue litmus solution to red colour. Hydrochloric acid reacts with zinc metal to produce zinc chloride and hydrogen gas. The reaction is given below: Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g) Hydrochloric acid reacts with Na2CO3 to produce carbon dioxide and turns the lime water milky as it forms calcium carbonate. The milkiness formed disappears when more than necessary carbon dioxide is passed through the solution. The reaction is as follows: Na2CO3(s/aq) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + CO2(g) Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l) CaCO3(s) + H2O(l) + CO2(g) → Ca(HCO3)2(aq) Rephrase but dont remove equations What is a base? A base is a substance that accepts protons or releases OH- ions when dissolved in water. They turn red litmus solution to blue colour. Sodium hydroxide (NaOH) reacts with zinc to produce sodium zincate and hydrogen gas. The reaction is given below: Zn(s) + 2NaOH(aq) → Na2ZnO2(aq) + H2(g) Sodium hydroxide (NaOH) reacts with solid sodium carbonate (Na2CO3) to produce sodium bicarbonate (NaHCO3) and sodium oxide (Na2O). The reaction is given below: Na2CO3(s) + 2NaOH(aq) → 2Na2O(aq) + H2O(l) + CO2(g) When NaOH reacts with lime water (Ca(OH)2), it forms a white precipitate of calcium carbonate (CaCO3) and water. The reaction is given below: Ca(OH)2(aq) + 2NaOH(aq) → CaCO3(s) + 2NaOH(aq) Overall, the properties of acids and bases can be studied by observing their reactions with indicators such as litmus, and with other substances such as metals and metal carbonates. |
| Chemical Required | Dilute hydrochloric acid (HCl) Dilute sodium hydroxide (NaOH) Zinc metal strips Solid sodium carbonate |
| Apparatus Required | Litmus paper (both blue and red) Test tubes Beaker Dropper Stirring rod |
| Procedure | 1 . Take three test tubes and label them as A, B, and C. 2 . Add 2-3 ml of dilute hydrochloric acid to test tube A. 3 . Add 2-3 ml of dilute sodium hydroxide to test tube B. 4 . Add 2-3 ml of water to test tube C as a control. 5 . Dip a red litmus paper in test tube A and a blue litmus paper in test tube B. 6 . Observe the color change on the litmus papers and record your observations in a table. 7 . Add a small piece of zinc metal to test tube A and B and observe any changes that occur. 8 . Record your observations in the table. 9 . Add a pinch of solid sodium carbonate to test tube A and B, and observe any changes that occur. 10 .Record your observations in the table. |
| Result | 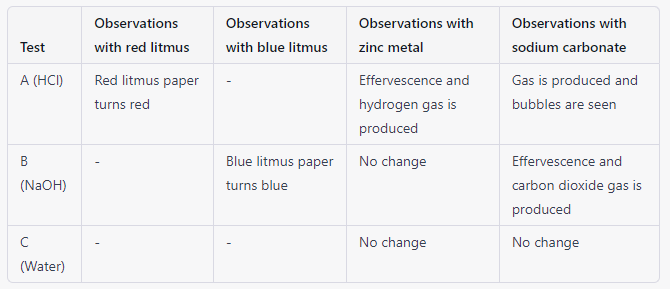 |
| Discussion | In this experiment, we have used dilute hydrochloric acid (HCl) and dilute sodium hydroxide (NaOH) to study the properties of acids and bases. When red litmus paper is dipped into HCl, it turns red, indicating that the solution is acidic. On the other hand, when blue litmus paper is dipped into NaOH, it turns blue, indicating that the solution is basic. When zinc metal is added to HCl, it reacts with the acid to produce hydrogen gas, which is seen as bubbles. When zinc metal is added to NaOH, no change is observed. When solid sodium carbonate is added to HCl, it reacts with the acid to produce carbon dioxide gas, which is seen as effervescence. When solid sodium carbonate is added to NaOH, it reacts with the base to produce effervescence and carbon dioxide gas. |
